Quick summary
You may have read recent cover stories about Wegovy (or semaglutide) and reduced heart attack risk. This news was perhaps not unexpected given previous positive evidence for positive health outcomes in patients using semaglutide for type 2 diabetes, but these results were nevertheless a seminal finding in the world of obesity care. We know scientific papers can be difficult to disentangle, though, so not to worry! We are here to highlight the key findings from the SELECT1 trial, the research trial that showed these incredible cardiovascular results.
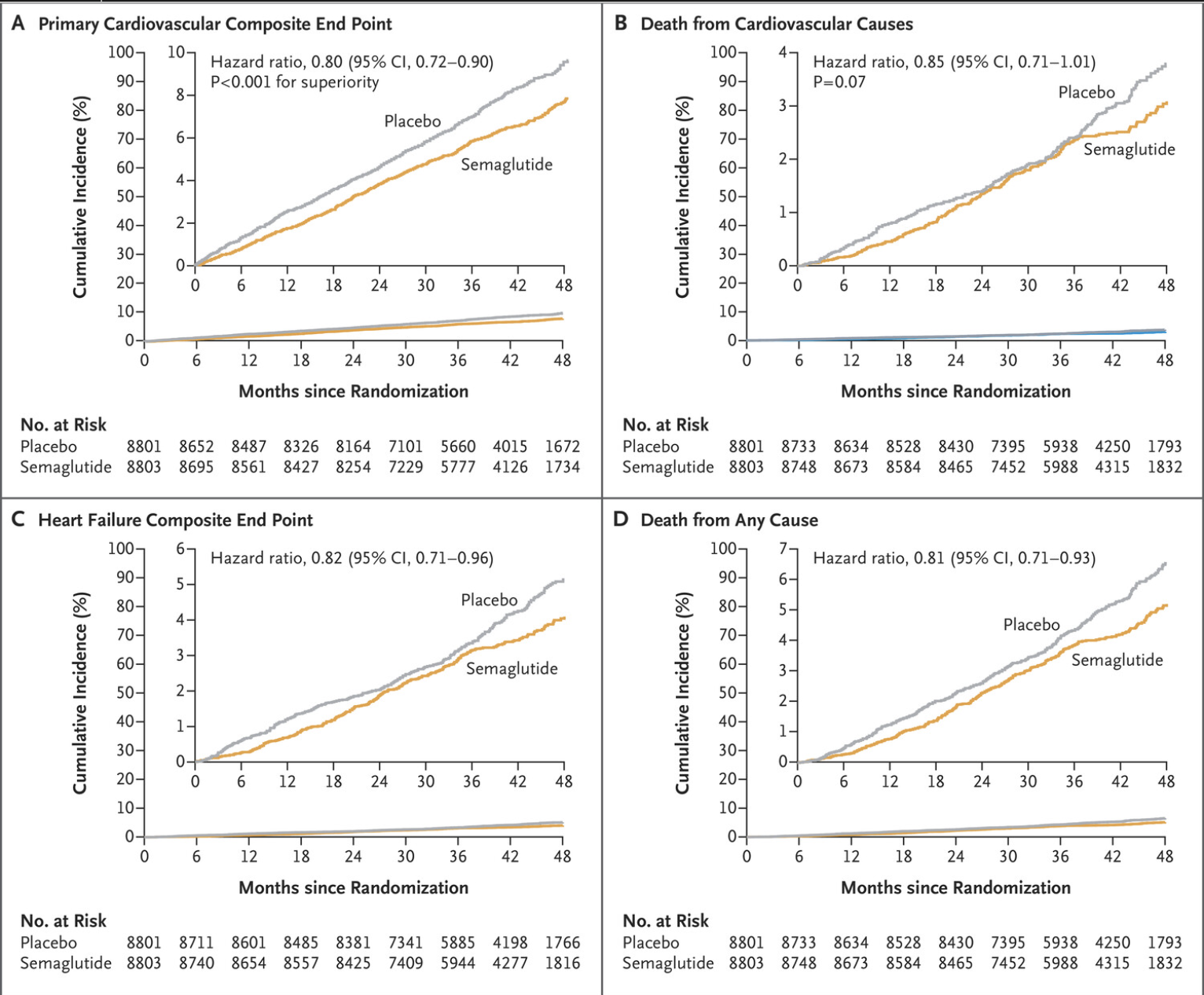
First things first—by what percentage did semaglutide reduce the risk of heart attack in patients taking the medication? In the SELECT1 trial, semaglutide (the compound in Wegovy) showed a 20% reduction in major adverse cardiovascular events (MACE) in 17,604 adults with pre-existing cardiovascular disease, overweight or obesity, without diabetes.
There were significant individual changes in body weight with semaglutide, with 67.8% of patients achieving 5% or more weight loss, and 44.2% achieving 10% weight loss at two years. Previous research has shown that with modest weight loss of 5%, patients can begin to see improvements in cardiovascular risk factors, hyperglycaemia, and quality-of-life measures (including personal well-being and physical functioning), whereas weight loss of greater than 10% is associated with reduced incidence of cardiovascular disease and improved mortality.
Cardiovascular benefits of semaglutide (Ozempic/Wegovy)
The most striking result from the SELECT trial is the 20% reduction in major adverse cardiovascular events (MACE), including heart attacks, strokes, and cardiovascular deaths, among participants treated with semaglutide. This included a substantial 28% reduction in heart attacks in patients as well as improved HbA1c, blood pressure and cholesterol levels. This emphasises the wide spectrum of benefit from semaglutide, not just for weight loss and establishes overweight and obesity as a modifiable risk factor for cardiovascular disease. Our full understanding of the cardioprotective mechanisms with semaglutide remains hypothetical, but it is likely secondary to the physiological benefits of reduced body fat and also the direct effect of semaglutide.

Wegovy is here! Start your free assessment
Mounjaro is here! Start your free assessment
Weight Loss Efficacy
Participants experienced an average weight loss of approximately 9.4% of their initial body weight in the SELECT1 trial after 24 months. This was lower than STEP-12, a study specifically investigating weight loss in people living with obesity being treated with semaglutide 2.4mg alongside intensive lifestyle intervention. However patients did not enrol in SELECT1 with the specific purpose of weight loss and only received standard lifestyle counselling relevant to lower cardiovascular risk and not losing weight. Demographics were also different in the two trials, with the SELECT1 trial containing an older, less female dominant, on average lower starting BMI and more comorbid population (that is, patients with a larger number of other conditions). Fewer patients on SELECT1 were on the maximum dose of semaglutide (2.4mg) as investigators were allowed to slow, decrease, or pause treatment, unlike the stepped dose escalation followed in STEP-12.
In a subset analysis3 on the long term weight loss effects in the SELECT trial, weight loss continued over 65 weeks and was sustained for up to 4 years, at which point semaglutide was associated with mean reduction in weight of 11.7%, waist circumference (−7.7 cm), and waist-to-height ratio (−6.9%) when compared to placebo (−1.5%, −1.3 cm and −1.0%).
Safety Profile
Safety remains a crucial consideration in any long-term therapy. The SELECT1 trial again confirmed that semaglutide is generally well-tolerated, with side effects primarily gastrointestinal in nature and consistent with those observed in previous trials. Overall, 16.6% of patients taking semaglutide discontinued treatment due to side effects compared to 8.2% with placebo.
This research provides robust evidence to support the prescription of semaglutide for patients at risk of cardiovascular events due to obesity. If utilised safely and in the correct patient populations, GLP-1 medications such as semaglutide will simultaneously reduce weight and cardiovascular complications, which could significantly improve quality of life and reduce healthcare burdens.
It is clear that the future of obesity treatment is being reshaped, promising better outcomes for patients and a new standard for therapeutic efficacy.
References
[1] Lincoff, A.M., Brown-Frandsen, K., Colhoun, H.M., et al. (2023). Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes. New England Journal of Medicine/the New England Journal of Medicine, 389(24), 2221–2232. https://doi.org/10.1056/nejmoa2307563
[2]Wilding, J. P., Batterham, R. L., Calanna, S., et al. (2021). Once-Weekly Semaglutide in Adults with Overweight or Obesity. New England Journal of Medicine/the New England Journal of Medicine, 384(11), 989–1002. https://doi.org/10.1056/nejmoa2032183
[3] Ryan, D. H., Lingvay, I., Deanfield, J., Kahn, S. E., et al. (2024). Long-term weight loss effects of semaglutide in obesity without diabetes in the SELECT trial. Nature Medicine. https://doi.org/10.1038/s41591-024-02996-7





